Heading 2
Why Conduct Research and innovation and the support available in NHS Western Isles?
NHS Western Isles recognise that facilitating research and innovation (R&I) activity is integral to gaining insights and developing services for the benefit of Western Isles patients. In addition conducting R&I activity can be an important element for the recruitment and retention of high quality staff at all levels. Anyone, including NHS Western Isles staff or others wishing to undertake health research or innovation projects in the Western Isles should read through the pages here for information on both the processes involved and the potential support available.
These pages contain information for anyone wishing to undertake research for the first time or for the more experienced researcher wishing to develop their research skills. Research can contribute to your continuing professional development (CPD) when focused on areas of service provision in your field.
Information about current R&I activity is also available and we can help you contact other researchers and developers with similar interests.
If you are thinking about conducting health-related research in the Western Isles, or have an innovative idea, perhaps related to your own service, which you would like to develop further, you are strongly encouraged to read through the information here and if wish to discuss further then contact Martin Malcolm, R&I Lead by clicking HERE.
Research Governance procedures and support in NHS Western Isles
As part of NHS Western Isles commitment to promoting high quality research, the Public Health Intelligence and Information Department, assisted by North of Scotland NHS Research node, will ensure research taking place within Western Isles NHS meets the requirements of the UK Policy Framework for Health Research and Social Care Research (2018, v3.3 07/11/17) and follow the principles of Good Clinicial Practice which are based on Articles 2 to 5 of the EU GCP Directive 2005/28/EC and the Medicines for Human Use (Clinical Trials) Amendment Regulations 2006.
Research governance procedures ensure:
- Recognised standards are upheld for research conduct
- Clear defined responsibilities of those involved in research
- Robust research quality and safeguards the public by:
- Enhancing ethical and scientific quality
- Promoting good practice
- Reducing adverse incidents and ensuring lessons are learned
- Preventing poor performance and misconduct
As part of this researchers are advised to consider recognized training modules in Good Research Practice and Good Clinical Research Practice which can be made available via the R&I Lead.
Research studies will therefore be assessed against a range of criteria including whether all necessary consents are in place as well as their potential impacts upon NHS work before receiving management approval for them to proceed in NHS Western Isles.
Are you considering undertaking research?
If so there are three initial steps for you to consider before starting your research study which are outlined below and assistance from R&I Lead is available:
Step 1: Is your study research or an audit/service evaluation?
Only research studies are required to follow full R&D governance processes including seeking R&D approval and potentially NHS ethical approval and not service audits or evaluations. However, it can often be difficult in determining precisely whether a project is research or service evaluation. Advice is available from the R&I Lead which is best to obtain at the outset of your project. There are a number of useful guides available to assist including the following decision tool produced by the UK NHS Health Research Authority Is my study research? (hra-decisiontools.org.uk).
A few key things to consider include the following:
Are your findings generaliseable?
Once you have considered this question and if you feel your study is research or are unsure then follow step 2 and register it on the NHS Western Isles Research register via the link below:
STEP 2: Registering your Research Study or a Study in which you are collaborating with external researchers.
It is important that any research being planned either by NHS Western Isles staff or external collaborators is registered with the R&D Lead on the R&D database using the approved form (view/download in word version or pdf version). This will include both research:
- By WI NHS Staff involving either WI or non-WI NHS premises and/or patients
- By non-WI NHS staff involving WI premises and/or patients or staff participants.
If you are either the Chief researcher for your own project or involved as Principal Investigator (PI) or local collaborator with external researchers then you should advise the R&D Lead of the project via the above form as soon as possible but certainly prior to study beginning. This will ensure you receive advice and guidance at the outset to the required processes and documentation required as part of the research approval process and where appropriate the necessary NHS ethical permissions. The R&D Management Approval process may require confirmation from your line manager and clinical superviser and/or Medical Director that you have access to appropriate researcher resources (inc. staff time) and follow relevant patient safety protocols where appropriate.
STEP 3: Registering Research studies and key documentation on national IRAS system.
IRAS is the Integrated Research Application System used for applying for permissions and approvals for health and social care/community research across the UK. Please read the information below before going through IRAS approval process.
IRAS:
- Generates the IRAS ID, which has been adopted by stakeholders across the UK as the common study identifier
- Enables you to enter the information about your project once instead of duplicating information in separate application forms
- Uses filters to ensure that the data collected and collated is appropriate to the type of study, and consequently the permissions and approvals required
- Helps you to meet regulatory and governance requirements
IRAS captures the information needed for the relevant approvals from the following review bodies
- Administration of Radioactive Substances Advisory Committee (ARSAC)
- Confidentiality Advisory Group (CAG)
- Gene Therapy Advisory Committee (GTAC)
- Health Research Authority (HRA) and Health and Care Research Wales (HCRW) for projects seeking HRA & HCRW Approval
- Medicines and Healthcare products Regulatory Agency (MHRA)
- NHS / HSC R&D offices
- NHS / HSC Research Ethics Committees
- Her Majesty's Prison and Probation Service (HMPPS)
- Social Care Research Ethics Committee
A useful guide to the potential approvals and consents you may require depending on the nature of your proposed research may be found at HRA website
Below is an introductory guide to the key steps to follow via IRAS:
Start off on your research project: You must apply as a new user on IRAS

Brief pathway of your research journey
- You must create an IRAS account.
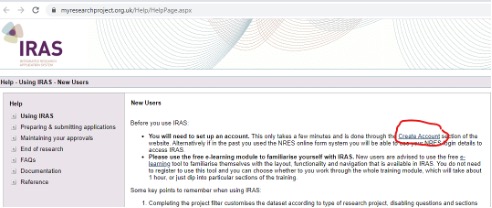
- You must start an application which will take you through a project filtration process:
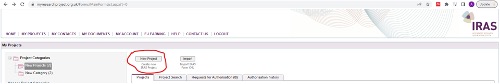
- Your project must be classified as research, audit, or evaluation (please read Step1 in guide above) on the project filter (see screenshot below) in order to further progress you application.
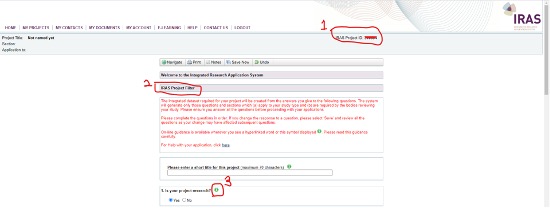
- Your application will close if you answer ‘NO’, i.e., your project is not research. There is an exception where your study is a health audit or service evaluation and requires Confidentiality Advisory Group (CAG) approval. This would be where your audit/evaluation intends to use identifiable patient data without explicit consent then you would select CAG in Q4 in the IRAS Project filter
- Your research application will progress if you answer ‘YES’, and you will be guided on all required documentation and approvals that are specific to your type of application.
- You may wish to refer to the IRAS Online Training module before starting off.
Should you require more information, kindly get in touch through the NHS Western Isles contact
NHS Western Isles recognises the value that research brings both to the health and care experience of its population but also for staff who may be keen to further develop their skills and knowledge in their areas of practice. This is particularly important for a remote island to ensure that the latest evidence supports the particular context of health care delivery in such a setting. Notwithstanding being a small island Health Board we seek to support researchers here in the following areas of research advice, training and funding:
ADVICE:
Advice on research is available in the first instance from the R&D Lead who may be able to assist around processes for undertaking NHS research including ethical and other approvals, links to relevant training, potential funding (see below) and other academic organisations and collaborations.
TRAINING:
There are key introductory courses on conducting health research particularly within the NHS that are available to staff online and provide assurance to those reviewing research that researchers have the required familiarity with the processes and considerations involved in undertaking such research.
Good Research Practice and Good Clinical Practice are recognised training modules to assist researchers in meeting the standards expected when undertaking research in an NHS organisation. This can be delivered online and takes just a few hours to complete or on occasion is available via face to face sessions with an experienced trainer. This is now increasingly becoming a prerequisite of research approval within the NHS and researchers are strongly advised to undertake this at an early stage in their research study planning.
Typical Course Content:
- Research Governance
- Roles and Responsibilities of those involved in Research
- Informed Consent
- Documentation and Quality Assurance
IRAS Online Training Module
IRAS is the principal electronic portal for applying to do research projects within NHS and assists with the necessary collection of documentation for receiving local R&D Management Approval and Ethical approval where required. An online module has been added to the IRAS website to guide new users through IRAS. The module, which is free, explains how IRAS is structured and guides users through how to prepare and make applications using IRAS. Go to https://www.myresearchproject.org.uk/ELearning/IRAS_E_learning.htm
However further support with IRAS is available from the R&D Lead if required.
FUNDING:
NHS Western Isles Endowment Funds may be available to fund Research and Development, and applications are welcome from NHSWI staff and/or external researchers looking to develop research in line with Western Isles research priorities.
Research Priorities are provided below although this is not an exhaustive list:
- Remote and Rural health
- Remote and Rural healthcare delivery
- Ageing population and Impacts on health and social care
- Approaches to management of Long term conditions
- Use of remote technologies in healthcare
- Administrative data and linkage in health research
CONTACT:
For Guidance on R&D governance arrangements, researcher support and registering your research study contact:
Martin Malcolm,
R&D Lead
Head of Public Health Intelligence and Information Department
Western Isles Hospital
Macaulay Road
Stornoway
Isle of Lewis.
HS1 2AF
E-mail: martin.malcolm2@nhs.scot
Tel: 01851 708254
USEFUL LINKS:
- Data Protection Act (1998) Information
- Download the Act
- NHS R&D Forum
- National Research Ethics Service
- Good Clinical Practice Directive
- North of Scotland Research Ethics Service
Medicines for Human Use (Clinical Trials) Regulations (2004)
Clinical Trials Toolkit:www.ct-toolkit.ac.uk
MHRA: http://medicines.mhra.gov.uk
- IT4Anxiety
- ChatPal
- mPower
- Online Arts Therapy
- NEXT PAGE: Staffing & Contacts
- LAST REVIEWED ON: January 20, 2025